Back to Florida Grade 5 Teacher Page.
Nature of Science
You can probably cover the activity on this page in a single class period, and it will lay a strong foundation for the rest of your year.
In 5th grade, students are exposed to many new concepts, especially in the Nature of Science. These include:
- Understanding the difference between experiments and other forms of scientific investigation.
- Understanding that scientific claims must be testable.
- Understanding variables and controls in experiments.
- Understanding the the difference between repetition and replication, and the function of each.
- Organizing data and using charts, tables and graphics to examine the data.
We also want to reinforce and expand on concepts that were introduced in earlier grades, including:
- Understanding that scientific claims must be based on evidence.
- The use of models in science.
Here is a quick, easy way to introduce these concepts while also covering the following standard:
SC.5.P.8.2: Investigate and identify materials that will dissolve in water and those that will not and identify the conditions that will speed up or slow down the dissolving process.
Vocabulary
- Observation: A scientific investigation where you record data but do not interfere or change anything yourself.
- Experiment: A scientific investigation where you test a hypothesis by changing a variable to see how it affects the results.
- Hypothesis: A tentative explanation for an observation, phenomenon, or scientific problem that can be tested by further investigation.
- Variable: Something that we could change that might change something in the results of our experiment.
- Repetition: Doing the same test or experiment multiple times to allow you to compare the data. Repetition lets us see patterns and can help spot variables that were not kept constant.
- Replication: This is when a different scientist/group of scientists try to exactly recreate a scientific investigation. If their results are the same, that supports the conclusions from the first investigation. If their results are different, that indicates that other variables may be involved.
Materials
- enough Lifesavers or other hard, sugar-based candy for each student to have at least 3 or 4 pieces
- a clock that displays seconds that all the students can see
- graph paper or a way to chart data on your whiteboard
- a glass of water
If you have students who cannot have sugar, have some sugar-free candy available. These students will be testing how quickly their candy dissolves compared to the sugar-based candy. Be sure to keep that data separate from the data on the sugar candy.
Proceedure
- Start by placing a clear glass of water at the front of the class. Drop in a piece of candy and ask your students what will happen as the candy sits in the water. Most classes will quickly decide that the candy will dissolve. Discuss that one kind of scientific investigation is observation, so you could test the class hypothesis (The candy will dissolve in the water) by waiting and watching to see what happens. By the time you have discussed this, quite a bit of the candy should have already dissolved. If you want to have the candy completely dissolve, show your students the What is Science? video while you wait.
- In science, we often need more information than we can get just by observation. For example, what if we wanted to find a way to get the candy to dissolve faster? Have students suggest things that would speed up the process of dissolving. Make a list on the board. Some of the suggestions will probably include:
- stirring the water
- breaking the candy into smaller pieces
- using hot water
- Explain that each of the things that affects how quickly the candy dissolves is called a variable. A variable is something that we could change that might change something in the results of our experiment. You can try a quick experiment to illustrate this. Be sure that all of the students can see the clock. When you give the students their candy, they will wait until you tell them to start before putting the candy in their mouth. They are going to test the hypothesis (What is a Hypothesis?) that breaking the candy into smaller pieces will cause it to dissolve faster.
For the first test, students will chew and crunch the candy into small bits. Remind students not to swallow the bits. We want them to dissolve. As soon as the last bit has dissolved, they should write down the time. After eating quite a few pieces of Lifesavers Hard Candy, Fruit Variety, I found that the average dissolve time for tiny bits was around one minute.
Once everyone has finished, write down the dissolve time for each student. Notice that all of the times are not exactly the same. Try plotting the data on a chart like the one pictured below.
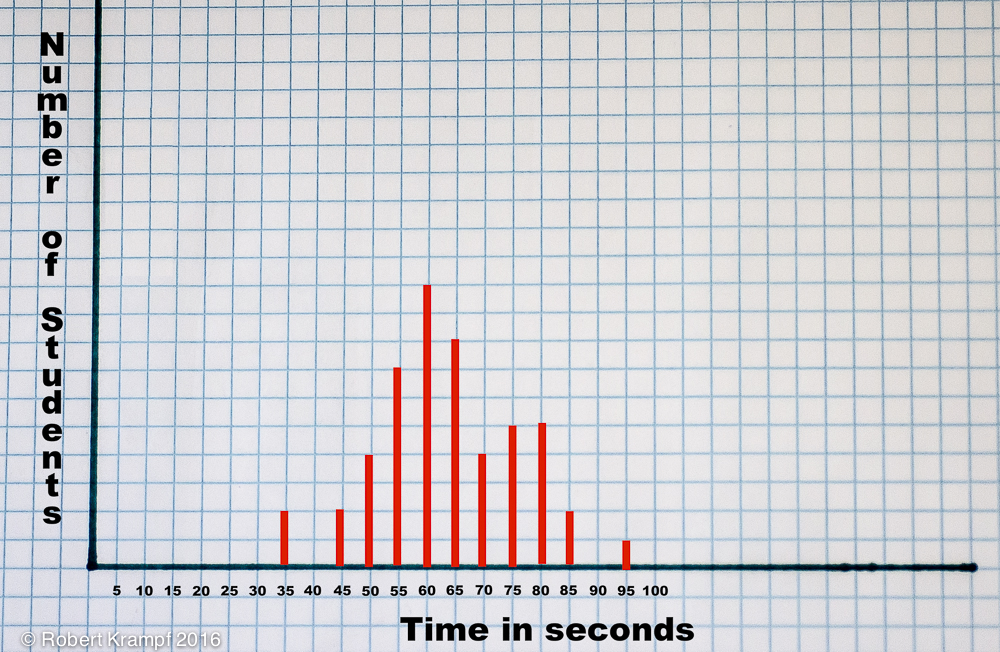
You can download a blank copy of the graph to print for yourself and your students.
This is why repetition (repeating your experiment several times) is important. Discuss why the times vary. Could there be other variables involved? Have students think of variables that might explain the difference. If they need prompting, suggest one of the following to start the process:
- Did some students swish the candy around more than others? This is similar to stirring the glass of water with the candy.
- Could some students have had more saliva in their mouth? More liquid could affect how quickly the sugar dissolved.
- Did some students crunch their candy into smaller pieces?
- Do some flavors dissolve faster than others?
- Were the candies exactly the same size?
Repetition can help us spot variables that we might have missed, and can sometimes show us patterns that we would miss otherwise.
Repeat the test, this time without chewing or crunching the candy. This will take longer (my average was six minutes and 15 seconds). Have students record the time when the last bit of candy has dissolved. Remind them that they are not supposed to swallow the pieces, and to hold them in their mouth until they completely dissolve. Tell them that if they can't resist a crunch, to make a note of that. Giving in to the temptation to crunch does not make them a bad scientist, as long as they record that crunch in their data. If they did not record it, it could cause the final data to be incorrect.
Make a second column on the board, and write the dissolve times for non-crunched candy. Discuss variations and potential variables that could have cause the differences. Plot the dissolve times on a separate chart.
Now it is time to evaluate the results to see if they support your hypothesis. Your results probably showed that crunching up the candy greatly reduced the dissolve time, so your results support your hypothesis.
This is a very good time to discuss that scientific testing does not "prove" things. Instead, the tests either support or do not support the hypothesis we are testing. Future tests might find that some other variable was the actual cause of the difference. Have students watch for news articles that claim that scientists have proven something, and discuss why this is not scientifically accurate.
At this point, you have covered quite a few of the Nature of Science standards. Your students have seen the difference between simple observation (candy in a glass of water) and experimental testing (crunched vs non-crunched dissolve rates.) They have identified variables. They have seen the importance of repetition, and used test data to look for patterns. They have organized their data using a chart to make it easier to see the patterns. Your students have evaluated their results to determine if they support their hypothesis.
You can easily add in the importance of replication by having other classes do the same investigation. Compare the results from several classes to see if they confirm your conclusions. Explain that since a different group of scientists (yes, in doing this your students are scientists) is doing the experiment on their own, they will not be influenced by the members of your class. If there is a large difference in the results, that would be a chance to look for some variable that was missed in the first experiment. If a variable was missed, that is NOT a bad thing. It means that your investigation found new evidence and learned something new. That is always a good thing in science, even if it means that your original hypothesis was wrong.
Where to go from here.
From here, you can follow whatever unit you would usually do, or you can dig deeper into SC.5.P.8.2 with more Nature of Science experimentation, leading into your unit on atoms to help students understand why each of the variables affects how things dissolve.
If you are able to spend some time on the Nature of Science, check out the Basic Concepts in Science Learnalong unit. This will take your students step by step through the Nature of Science standards. The unit can also be used for background reading to get ideas for adding Nature of Science activities into your other units.
